Guest Editor
Ivan Rusyn, MD, PhD, Texas A&M University, USA
Terry R Van Vleet, PhD, AbbVie, USA
Submission Status: Open | Submission Deadline: 19 May 2025

Ivan Rusyn, MD, PhD, Texas A&M University, USA
Terry R Van Vleet, PhD, AbbVie, USA


Ivan Rusyn, MD, PhD, Texas A&M University, USA

Terry R Van Vleet, PhD, DABT, ATS, ERT, AbbVie, USA

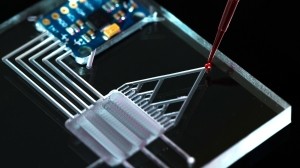
Microphysiological systems are breakthrough innovations to recapitulate aspects of complex human physiology in vitro and facilitate more precise clinical prediction compared to previous models. These systems are created by merging several threads of science and technology in pharmacology, toxicology, and biomedical engineering and exist in different forms from an Organ(s)-on-a-Chip (OOC) to other Complex in vitro Models (CIVM). These innovations represent a potential paradigm shift in how we model human biology in vitro, offering opportunities to mimic the complexities of human organs and tissues. Through the integration of these devices/technologies into both research and drug development, researchers may uncover novel pathways for drug discovery, disease modeling, and toxicity assessment.
These advancements serve to establish more efficient, ethically grounded, and predictive methodologies. Future developments target to scale up production, enhance complexity for multi-organ interaction modeling, and broaden applications for personalized medicine and high-throughput drug screening. These efforts hold potential for transforming preclinical research and improving the accuracy of predicting human responses to pharmaceuticals and environmental toxicants.
We invite toxicologists, biomedical engineers, and interdisciplinary researchers to explore the recent advancements in and examples of the utility of OOC and CIVM and contribute their robust science to this Collection. Key topics of interest for this Collection include, but are not limited to:
This Collection supports and amplifies research related to SDG 3: Good Health and Well-Being.
Image credit: © [M] luchschenF / Stock.adobe.com
There are currently no articles in this collection.
This Collection welcomes submission of original Research Articles. Should you wish to submit a different article type, please read our submission guidelines to confirm that type is accepted by the journal. Articles for this Collection should be submitted via our submission system, Snapp. During the submission process you will be asked whether you are submitting to a Collection, please select "Advances in microphysiological systems and organs-on-chips technologies" from the dropdown menu.
Articles will undergo the journal’s standard peer-review process and are subject to all of the journal’s standard policies. Articles will be added to the Collection as they are published.
The Editors have no competing interests with the submissions which they handle through the peer review process. The peer review of any submissions for which the Editors have competing interests is handled by another Editorial Board Member who has no competing interests.